Tag: COVID-19
-
EHPVA Does Not Administer COVID19 Vaccines
Please Take Notice EHPVA continues to practice medicine in service of all our patient’s medical needs. We appreciate the enthusiasm of our patient’s desire to fight the COVID19 pandemic and Stop the Spread. However, our small office cannot answer all questions and concerns about COVID19 and continue administering to our patients’ needs at the same…
-

Dr. Kartchner – Guest Update on COVID-19
A quick note from Dr. Kanal: Curbside testing for Coronavirus is available through Piedmont Urgent care, but does require physicians’ orders I do believe that is for symptomatic patients. More information to come. UPDATE 5/3/2020 https://www.who.int/news-room/commentaries/detail/immunity-passports-in-the-context-of-covid-19 In summary, we are deferring universal antibody testing for the moment until we have better data on which assays…
-

COVID-19 Update: April 7th
It’s time for another installment of our coronavirus updates! I wish what I am about to write was a belated April Fools’ joke, but alas, we are in a strange new reality. As expected, many things have changed since my last posting. DC, MD, and VA have all imposed formal “stay at home” orders with…
-
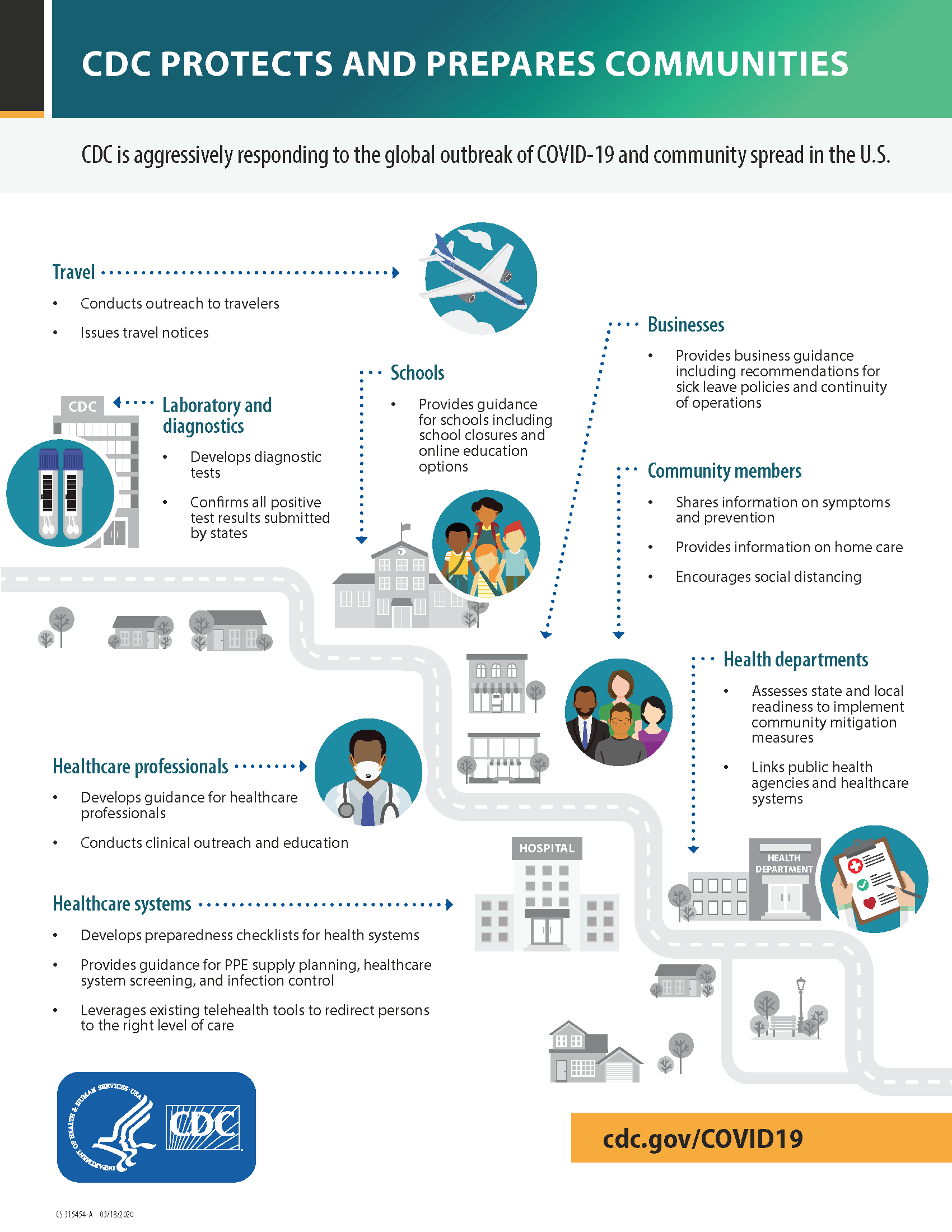
COVID-19 Update: March 26th
The following letter is a reprint State Health Commissioner’s Treatment of COVID-19 Letter sent to Clinicians on March 25th: Treatment of COVID-19 March 25, 2020 Dear Colleague: In the most recent days, there has been a surge in demand of potential treatments for COVID-19 for drugs commonly used to treat malaria, lupus, rheumatoid arthritis, HIV,…
-

COVID-19 Update: March 24th
The following post is a reprint of an article from Duke Antimicrobial Stewardship Outreach Network a (Duke Antimicrobial Stewardship Outreach Network, 2020) Should non-steroidal anti-inflammatory drugs (NSAIDS) be administered to patients who have COVID-19? On March 11, a correspondence published in Lancet Respiratory Medicine suggested theoretical harm related to the use of angiotensin-converting enzyme inhibitors (ACEi),…
-
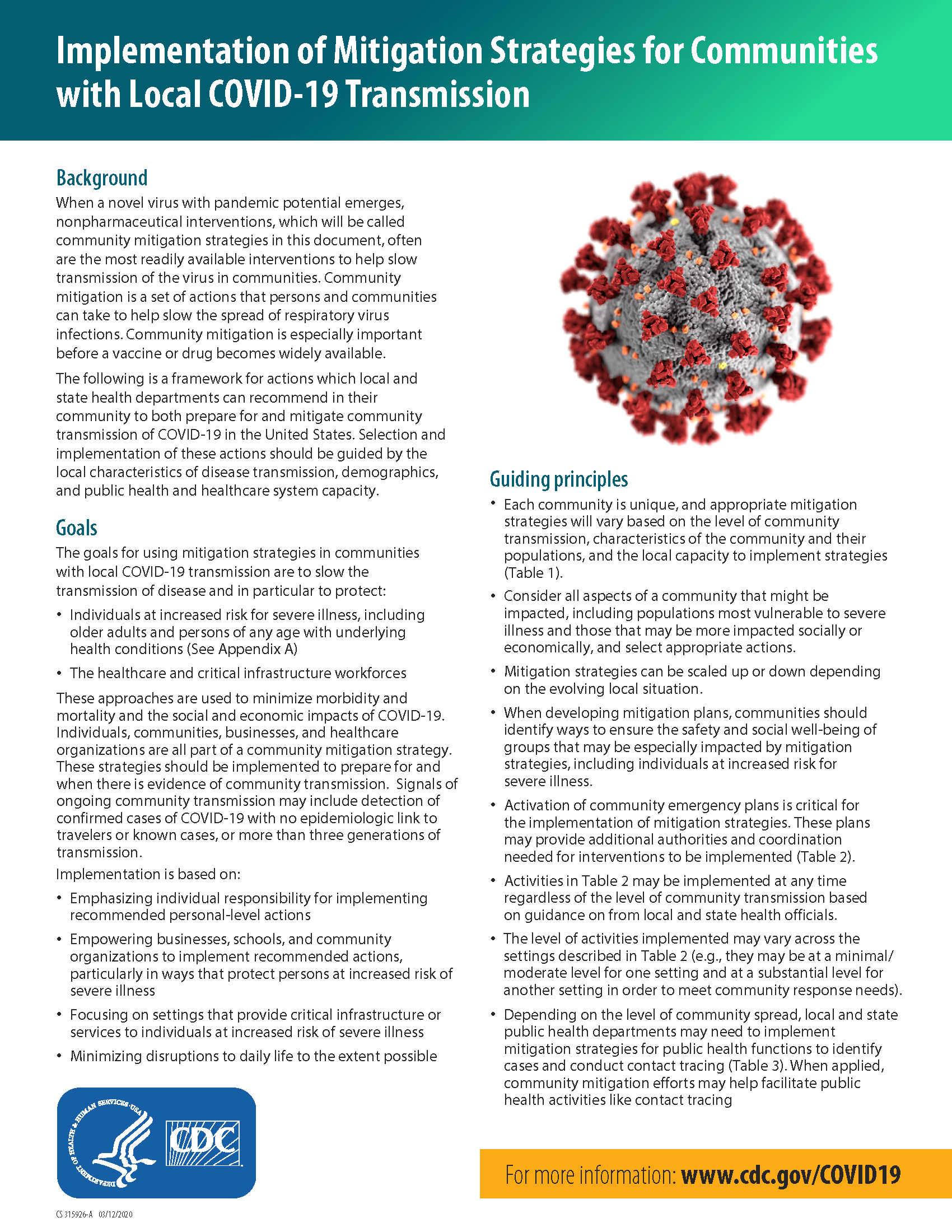
COVID-19 Update & Editorial: March 23rd
Good day, dear patients! A few of you have submitted questions about the recent statements in azithromycin and Plaquenil (hydroxychloroquine). Please note that some of what is stated below is my personal opinion and not formal guidance. I have included source links where applicable and, as always, encourage you to continue to use the CDC…
-
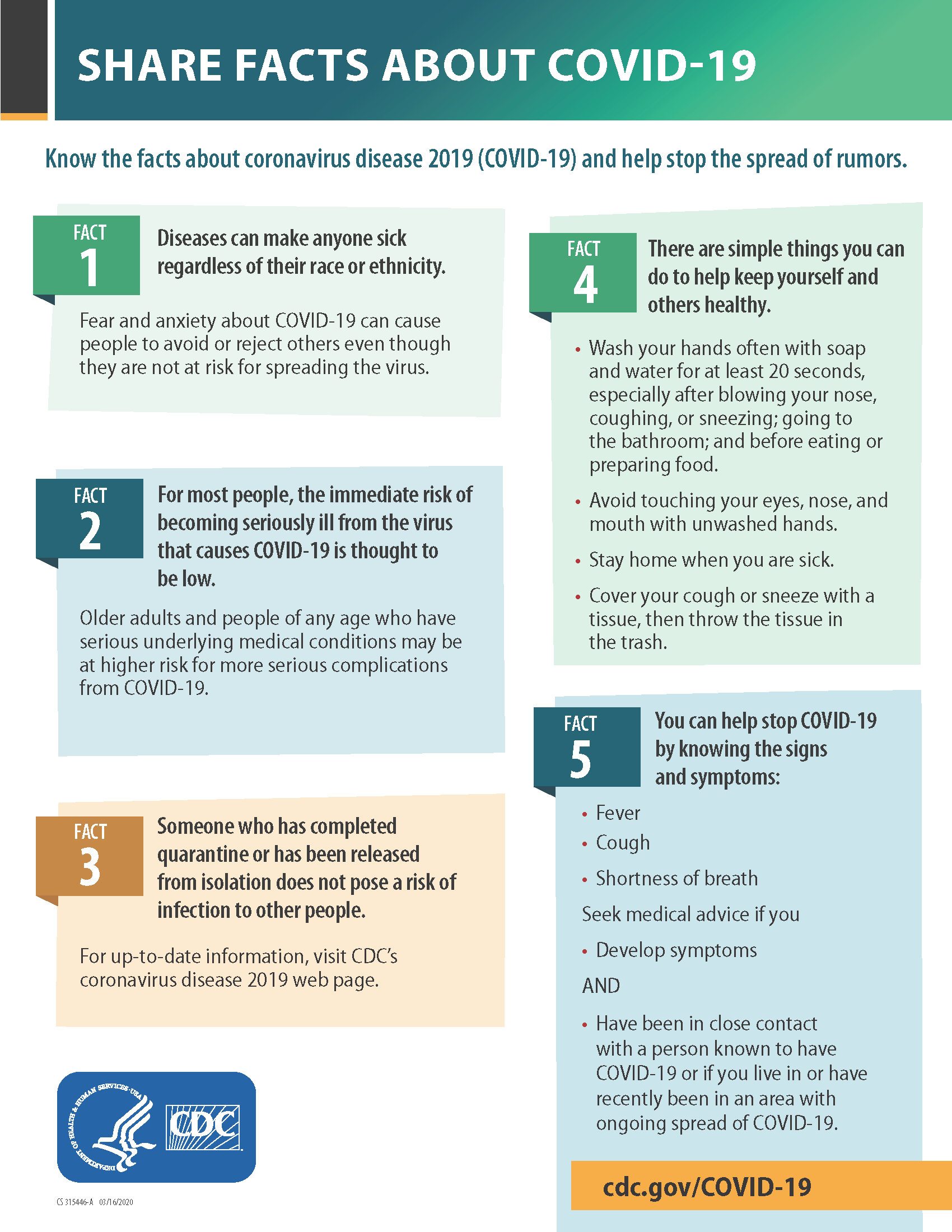
COVID-19 Update: March 20th
Patients, As of 3/17/2020, EHP has moved primarily to telemedicine visits for anything routine. Please refer to the FAQ from our 3/17/2020 update to get more information on how this process works. To limit exposure of patients and staff, EHPVA will be operating under limited office hours. Dr. Kanal will be in-office, with those reduced office…
-
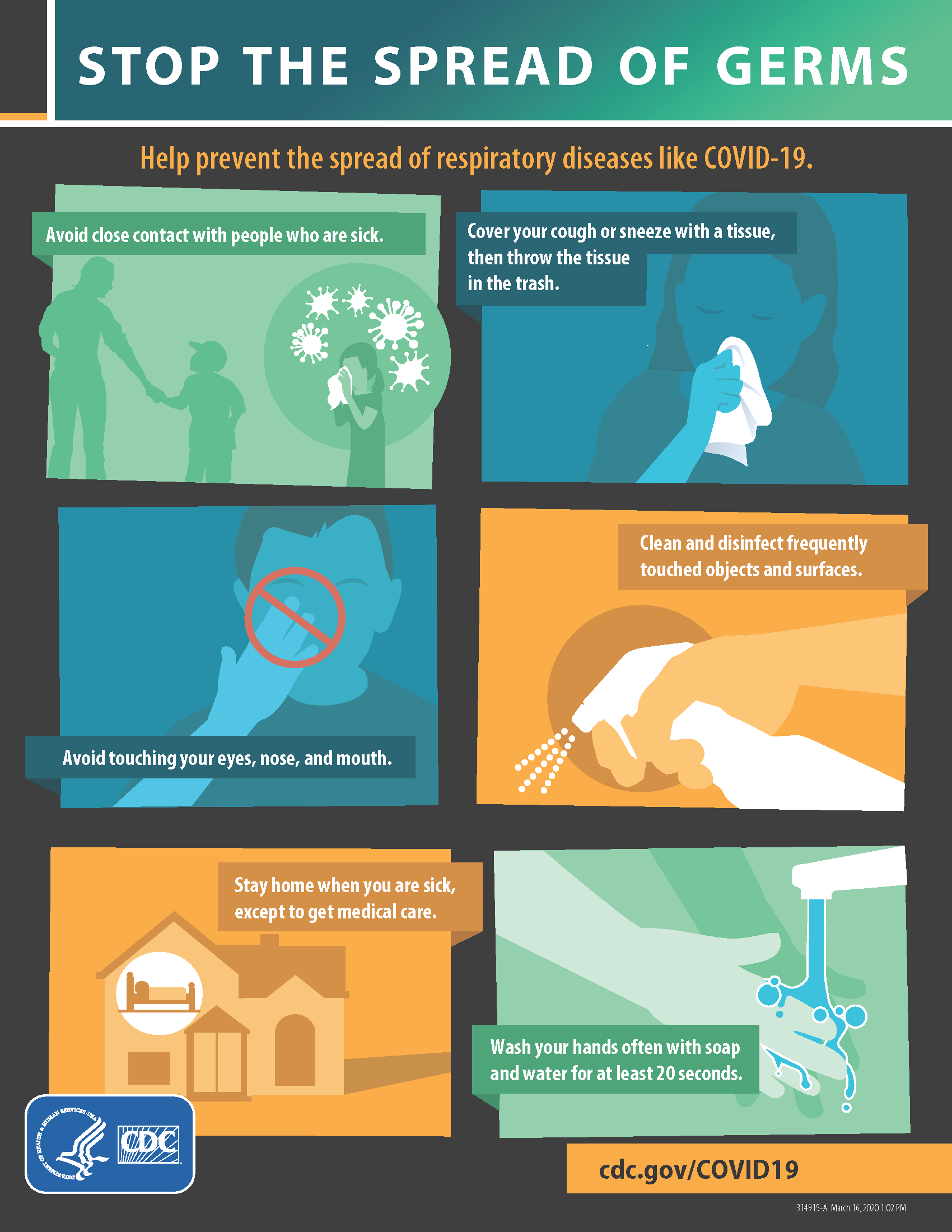
COVID-19: Update March 17th
Patients, I have been in contact with the local health department. They are not referring patients to their PCP for COVID-19 testing. Though commercial tests are being generated, these are only in limited supply. We do not have these tests available for mass testing. Please contact us to be evaluated by phone if you have…
