The following letter is a reprint State Health Commissioner’s Treatment of COVID-19 Letter sent to Clinicians on March 25th:
Treatment of COVID-19
March 25, 2020
Dear Colleague:
In the most recent days, there has been a surge in demand of potential treatments for COVID-19 for drugs commonly used to treat malaria, lupus, rheumatoid arthritis, HIV, bacterial infections and other conditions. This is leading to an inadequate medication supply for patients already taking these medications for chronic conditions and hospitalized COVID-19 patients being treated with these medications under facility-specific treatment protocols while studies are ongoing.
There are currently no antiviral drugs approved by the U.S. Food and Drug Administration (FDA) to treat COVID-19. Some in-vitro or in-vivo studies suggest potential therapeutic activity of some agents against related coronaviruses, but there are no available data from observational studies or randomized controlled trials in humans for the CDC to support recommending any investigational therapeutics for patients with confirmed or suspected COVID-19 at this time.
The Virginia Department of Health in consultation with the Virginia Department of Health Professions recommends the following:
- Prescriptions for chloroquine, hydroxychloroquine, mefloquine and azithromycin should be restricted in the outpatient setting and should require a diagnosis “consistent with the evidence for its use.”
- Community pharmacists should use professional judgement to determine whether a prescription is valid and that there is a bona fide practitioner-patient relationship prior to dispensing.
- Prioritize treatment for continuation of existing medication therapy, inpatient settings, and other indications where there is not an alternative therapy.
- Advise against hoarding these medications or stockpiling.
There is currently no available data from randomized clinical trials to inform clinical use. Refer to the CDC for more information on therapeutic options for COVID-19. (https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html).
Sincerely,
M. Norman Oliver, MD, MA
State Health Commissioner
A version of this letter is available on the VDH Resources for Health Care Professionals web page.
Further Updates on COVID-19 from the VDH
- CDC recently released the Coronavirus Self-Checker to help people make decisions about seeking appropriate medical care. This system is not intended for the diagnosis or treatment of COVID-19 or other diseases.
- Despite expanding testing to more private laboratories and increasing capacity at participating laboratories, the demand for testing far
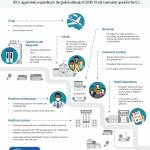
CDC is aggressively responding to the global outbreak of COVID-19 and community spread in the U.S. exceeds the supply. Public health testing at Virginia’s state laboratory, the Division of Consolidated Laboratory Services (DCLS), is reserved for symptomatic people who meet the recently updated VDH priority investigation criteria.
- To expedite approval for public health testing at DCLS, VDH developed an online COVID-19 Testing Request Form for healthcare personnel (HCP). After submitting the request, HCP will receive an email confirming receipt. VDH staff will monitor this system Monday through Sunday from 9 am to 5 pm and will provide a testing approval decision within three hours for requests received during this period. If the patient is being discharged in the meantime, you may proceed with specimen collection. VDH approval is not required to collect a specimen.
- For COVID-19 testing at DCLS, collect one nasopharyngeal swab in viral transport media. Updated instructions are available on the DCLS website. Do not ship specimens without prior VDH approval.
- For patients being tested at a laboratory other than DCLS, contact the laboratory for details about testing availability and instructions. VDH approval is not required for testing at these labs.
- Regardless of testing status, please provide this VDH patient handout for people with confirmed or suspected COVID-19 who are being discharged. It is critical to instruct all those with suspected or confirmed COVID-19 to stay home, even if symptoms are mild, unless in-person medical evaluation and care are required.
- Virginia’s local health departments do not provide primary care and thus are not equipped to clinically evaluate patients with respiratory symptoms. Please do not refer your patients to a local health department for testing.
- A positive COVID-19 test result from DCLS or another laboratory is considered confirmed for case counting purposes. Confirmatory testing at CDC or another lab will not be performed.
Click Here for CDC Information on COVID-19
Click Here for VDH Information on COVID-19
Click Here for Coronavirus.gov
Missed an update? Click Here to visit our ongoing Coronavirus updates page.